Mechano-medicine
Targeting Cardio- and Cerebro-vascular Diseases
Why vascular mechanobiology?
Abnormalities in blood vessel and blood flow properties serve as key drivers of severe cardiovascular and cerebrovascular pathologies and cancer. Fundamentally, aside from genetics and environmental factors, such diseases stem from cellular and tissue dysfunction during their adaptation to mechanical, biochemical, and topological cues. For example, endothelial tissues lining healthy arteries adapt to laminar blood flow to maintain a selective barrier that is key to physiological vascular function. However, in aging-related atherosclerosis or stroke, blood flow is no longer laminar. Here, turbulent flow results in endothelial cell junction rupture, increased tissue permeability and hence, vascular dysfunction.
Our vision is to decode, predict, and engineer disease-relevant mechano-responses of the endothelium. We will provide crucial mechanistic insight into how long timescale, force-dependent gene expression drives rapid biochemical signaling and phenotypical behaviors to ultimately tune vascular tissue function across scales. Decoding these precise mechano-chemical feedback loops will help establish models to tune tissue function and bridge the gap between the fields of biology and medicine.
Video: Dynamics of force-sensitive protein FHL2, and the microtubule network visualized in a migrating aortic endothelial cell.
Endothelial Mechanobiology in Cardiovascular Aging
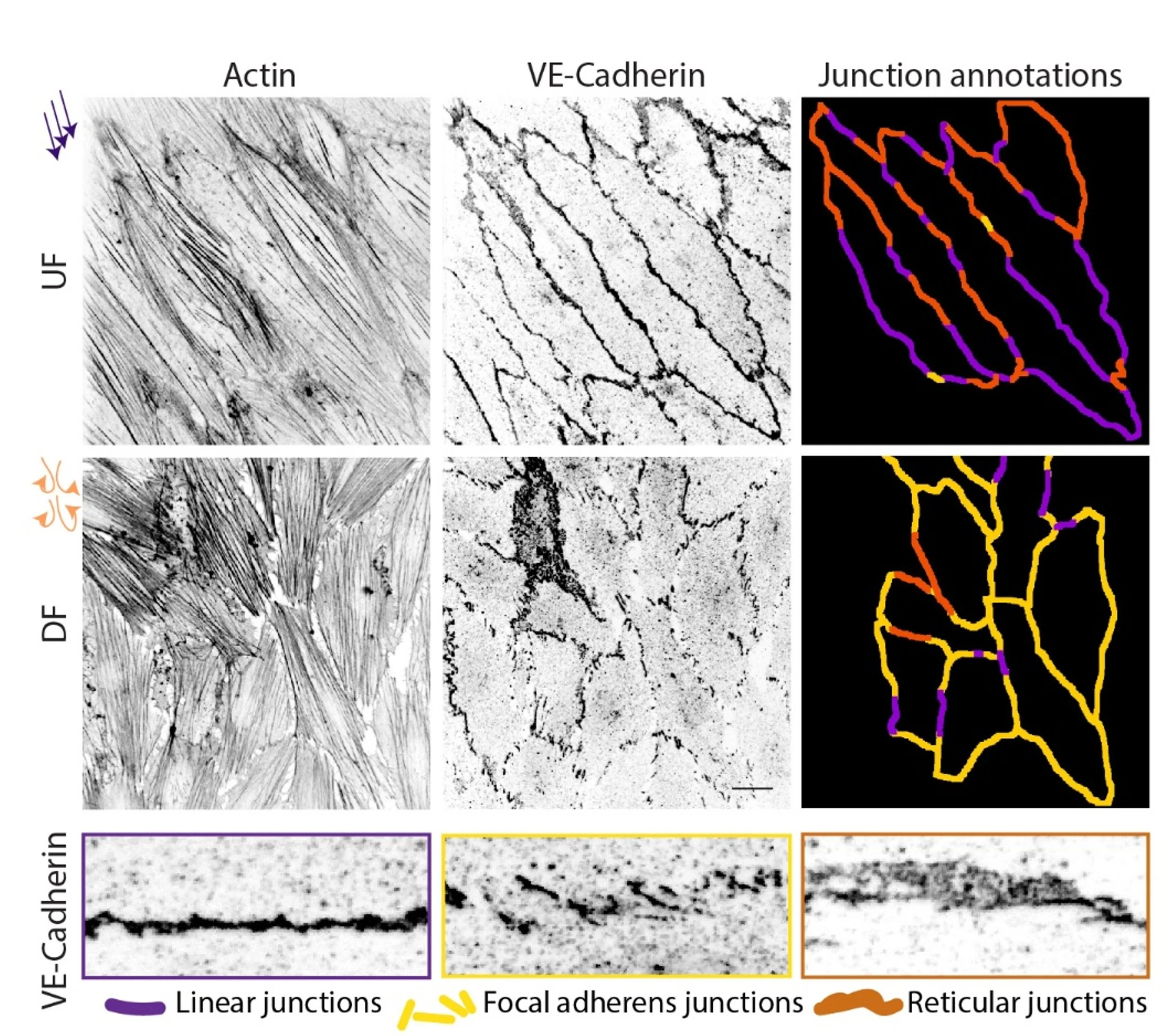
Endothelial tissues in blood vessels constantly adapt to blood flow-derived forces by polarizing their cytoskeleton, adhesions, and organelles, all while maintaining cell-cell interactions and selective barrier function; this barrier function is lost in aging-related diseases like atherosclerosis. Using bioengineering and biophysical approaches, we can show in vitro that cell junction disruptions occur under disturbed flow mimicking atherosclerosis (see Figure). However, the mechanisms of such drastic tissue remodeling remains unclear.
Here, using in vitro approaches as well as patient heart tissues, and leveraging advanced high-resolution microscopy, multi-omics approaches, biochemistry, and bioengineering tools, we will decode the mechano-chemical feedbacks driving vasular tissue dysfunction. Specifically, we will focus on how transcriptional regulation of force-sensitive genes, cytoskeletal crosstalk, and cell junction remodeling coordinately tune the mechanoresponse of endothelial tissues.
Image: Cell-cell junctions marked by VE-Cadherin and the actin cytoskeleton in human aortic endothelial cell monolayers experiencing healthy unidirectional flow (UF) or disturbed flow (DF) mimicking atherosclerosis. Cell junction morphologies represent three functionally distinct classes as shown, where focal adherens junctions seen in response to perturbed flow drives high permeability and tissue dysfunction.
Vascular Growth and Remodeling in Cancer
A key hallmark of several tumors is the process of neovascularization and angiogenesis mediated by endothelial cells. Although angiogenesis is necessary during development, repair and maintenance of the vasculature, this process can also be exploited by tumor cells for invasion and metastasis. However, the mechanisms of vascular growth and remodeling supporting tumor invasion remains unknown.
In primary and metastatic gliomas, the endothelial blood-brain barrier is barrier (BBB) is highly permeable, and is spatially surrounded by migratory glial cells including reactive astrocytes and tumor cells. Our goal is determine the mechano-chemical mechanisms mediating endothelial remodeling, specifically angiogenesis and neovascularization, in driving gliomas and other cancers. Here, we will leverage 2D and 3D models of tumor invasion and vasculature to map the mechanisms of cell adhesion and migration, spatial interaction of different cell types, and the transcriptional control of force-sensing and adaptation in angiogenesis-mediated tumor progression.
Video: Angiogenesis of endothelial cells in 3D visualized by the cell membrane (orange). Cell migration, precise cell-cell junction remodeling, and cell proliferation are essential for efficient angiogenesis.
Machine learning models for tissue mechanobiology
With mechanobiology insights and the power of computational approaches, we are integrating novel biology to build data-driven modeling of tissue function. In this context, we are developing and combining innovative wet-lab approaches (from cell biology and biochemistry to biophysics), with computational AI/ML modeling and data science, with the ultimate goal of understanding the mechanisms of endothelial mechanoresponses. Specifically, combining microscopy imaging, sequencing, and quantitative cell biology approaches, we will bridge across scales to develop machine learning models for vascular disease prediction.
Image: Integration of systems biology and machine learning to develop models for vascular tissue function in health and disease.